Treatment of Sleep Apnea
How is Sleep Apnea Commonly Treated?
Sleep Apnea is commonly treated with oral dental devices, mandibular advancement devices, the Inspire device, CPAP machines, and Nightlase laser therapy.
We Use Rescue Laser Therapy
Because approximately 94% of patients who have Sleep Apnea suffer from Snoring, and Snoring represents a physical obstruction during nighttime breathing, we believe NightLase® Laser Therapy is an intelligent, ideal, FDA approved therapy for Snoring.
Additionally, NightLase® Laser Therapy is an ideal Rescue Treatment for those individuals who simply can not tolerate or refuse to wear the CPAP mask as the CPAP is very un-natural and uncomfortable.
To Learn More about NightLase® Laser Therapy: CLICK HERE
Key features of NightLase® Laser Therapy include the following:
- FDA-Approved
- No Pain
- No Down time
- No Side Effects
- No Anesthesia required
- Non-Ablative
- No Cutting
- No Burning
- Highly Effective Success Rate
- In-Office Procedure (25 minutes); Return to Normal Activity IMMEDIATELY
- Safe
- Initiates Conversion of Existing Collagen to More Elastic and Organized Forms of Collagen
- Induces Neocollagenesis which is the creation of New Collagen by Fibroblast cells
- Does NOT require a device to be worn on your face during sleep
- Does NOT require a device stuffed in your mouth during sleep
- Involves No chemical treatments
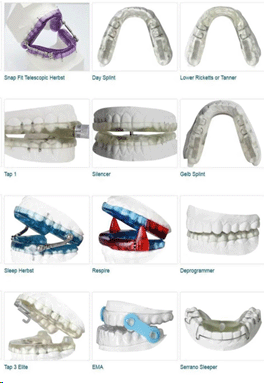
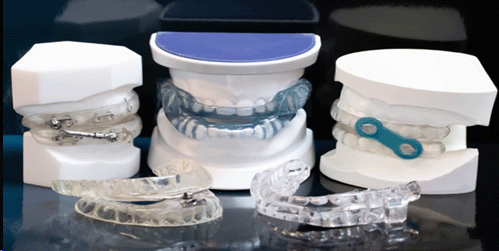
Oral Dental Devices and Mandibular Advancement Devices
These are frequently used and are markedly uncomfortable. Often times it takes a trial of a number of different oral dental devices or mandibular advancement devices to get a correct “fit” in the mouth of an individual.
Many patients report difficulty with falling asleep and staying asleep when something is stuck in their mouth.
Inspire
There are 2 wires which are implanted and connected to a stimulator device.
One wire is connected to your chest and another wire is placed in your neck which is wrapped around one of the nerves which stimulates your tongue.
When your chest moves – it stimulates the implantable device which in-turn stimulates the second wire so that it electrically makes your tongue move.
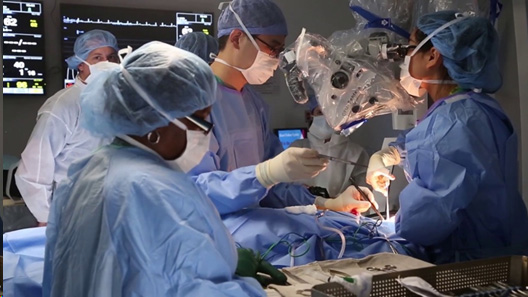
There are MANY CONCERNS regarding the Inspire device – first is the COMPLICATION RATE.
In scientific studies, as reported in the American Journal Otolaryngology, the most commonly reported adverse events to patients who received the Inspire were:
Infection (n = 50, 34.2%)
Neuropraxia (n = 22, 15.1%) (nerve injury commonly induced by focal demyelination or ischemia)
Hematoma/ Seroma (n = 17, 11.6%)
RE-OPERATION: A total of 83 adverse events (42.35%) Required RE-OPERATION
The most common Re-Operation performed for adverse events to patients with Inspire were EXPLANTATION (n = 30, 46.2%) (DEVICE REMOVAL)
Device repositioning/lead revision (n = 24, 36.9%).
The most common RE-OPERATION performed for DEVICE MALFUNCTIONS was Device Replacement (n = 10, 55.6%).
This data is reported by Bestourous DE, Pasick LJ, Benito DA, Zapanta PE. Adverse events associated with the Inspire implantable hypoglossal nerve stimulator: A MAUDE database review. Am J Otolaryngol. 2020 Nov-Dec;41(6):102616. doi: 10.1016/j.amjoto.2020.102616. Epub 2020 Jun 25. PMID: 32645535.
There are also MANY other ADVERSE CONSIDERATIONS in using the Inspire:
Implantable Cardiac Device Malfunction (Pacemakers; Defibrillators): from the manufacturer: “the electrical pulses from the Inspire system could affect the ability of the cardiac device to sense and respond to heart function as intended.”That means, if your Cardiologist has placed either a pacemaker or defibrillator in you – they may May NOT Work properly !!!This, of course, is ENORMOUSLY Dangerous and can be potentially Lethal.
MRI: Many patients can no longer get an MRI, depending on the model of Inspire used.
Electromagnetic Interference: Because the inspire implant works through electromagnetic waves, the following equipment or environments could generate enough electromagnetic “disturbance to potentially create unwanted stimulation” from the Inspire Device.
The Inspire manufacturer states these should be AVOIDED:
- Large stereo speakers
- Magnets
- Antennas of citizen band (CB) or ham radios
- Electric induction heaters
- Microwave communication transmitters
- Television and radio transmitting towers
- Power lines or power generators
- High-voltage areas
- Equipment used for decreasing or eliminating magnetic fields
- High-power amateur transmitters
The Inspire manufacturer states these should be used with CAUTION:
- Mobile phones
- Tablet computers
- AM/FM radios
- Cordless and conventional telephones
- Computer disk drives
Battery Life: The function of the implantable Inspire device (its “Stimulator”) depends on its battery. Battery life depends “significantly” on “the number of hours you have therapy on and the therapy settings.”
CPAP: Visit this page to learn more about CPAP and its Failures.
